-

 2022/05/13
2022/05/13Announcement on Early Termination of GV-971 Global Multi-Center Phase III Clinical Trial
GV-971
-

 2022/01/13
2022/01/13Green Valley Obtains IND Approval from U.S. FDA for Sodium Oligomannate’s Global Phase II Clinical Trial in Treating Parkinson’s Disease
GV-971
-

 2021/12/03
2021/12/03GV-971 Gets Included in China National Reimbursement Drug List
GV-971
-

 2021/06/16
2021/06/16GV-971 Global Phase III Clinical Trial Officially Initiates in Greater China to Clarify Its Potential in Changing Disease Course
GV-971
-

 2021/06/04
2021/06/04Nature Index-China: Alzheimer’s drug trial explores gut-brain link
GV-971
-

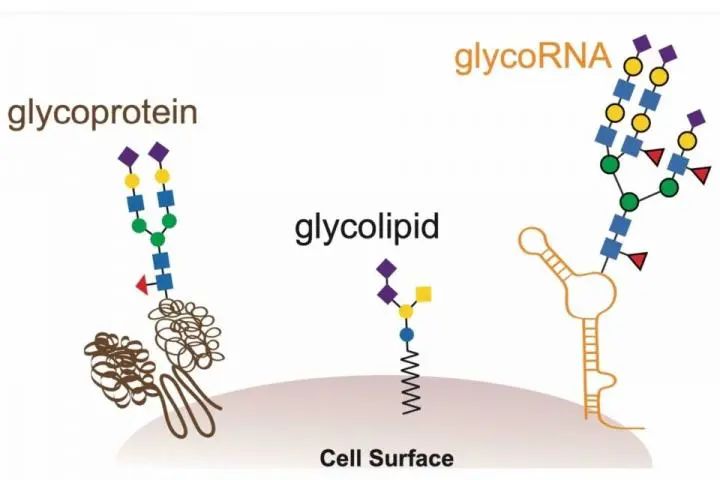 2021/05/18
2021/05/18Cell-Stanford University Beyond the Textbook Pattern: A Brand-new Biomolecule!
GV-971
-

 2021/04/19
2021/04/19Alzheimer’s Disease Care Project Initiates to Provide Long-term Benefits to Patients
GV-971
-

 2021/02/08
2021/02/08GV-971 Global Phase III Clinical Trial Completes First Patient Dosed Globally
GV-971
-

 2020/11/06
2020/11/06GV-971 Global Multi-center Phase III Clinical Trial Kicks-off Patient Enrollment Process
GV-971
-

 2020/04/26
2020/04/26Green Valley Obtains IND Approval from U.S. FDA for GV-971 Global Phase III Clinical Trial
GV-971
-

 2020/01/21
2020/01/21GV-971 Was Awarded Top10 Novel Drugs in the 13th Health China Forum
GV-971
-

 2019/12/29
2019/12/29GV-971 Officially Launched in China
GV-971
-

 2019/12/23
2019/12/23Nature Announced Ones to Watch in 2020 and Meiyu Geng Got Selected
GV-971
-

 2019/11/02
2019/11/02Green Valley Announces NMPA Approval Of Oligomannate For Mild To Moderate Alzheimer's Disease
GV-971
-

 2019/09/07
2019/09/07Cell Research: Latest Progress of the Drug Reshaping Gut Microbiota to Inhibit Alzheimer Disease in Mice
GV-971
-

 2019/09/06
2019/09/06GV-971 Novel Mechanism of Action Results Featured on the Cover of Cell Research
GV-971
-

 2018/10/26
2018/10/26Green Valley Announced Promising Findings from a Phase 3 Clinical Trial of GV-971
GV-971
Your location: