GV-971 China Clinical Study
1199 subjects participated1
Phase III was conducted at 34 grade A tertiary hospitals nationwide led by Shanghai Mental Health Center and Peking Union Medical College Hospital, with 818 subjects participating
Managed by IQVIA, a global leader in new drug R&D services1
China Phase III Clinical Study
(ClinicalTrials.gov Identifier:NCT02293915)
Results of a 36-week China phase III clinical study showed that:
GV-971 improved the cognitive function of patients consistently and significantly throughout the observation period2,3
Significant improvement was observed in cognitive function, the main efficacy indicator, compared to the placebo group, with an improvement of 2.54 points in the cognitive function scale (ADAS-Cog) score (p<0.0001)
Fast-acting and consistent improving on cognitive function in patients
Excellent safety profile with an adverse event rate comparable to the placebo group
-

Significant and consistent improvement on cognitive function throughout the trial from week 4 onwards
-
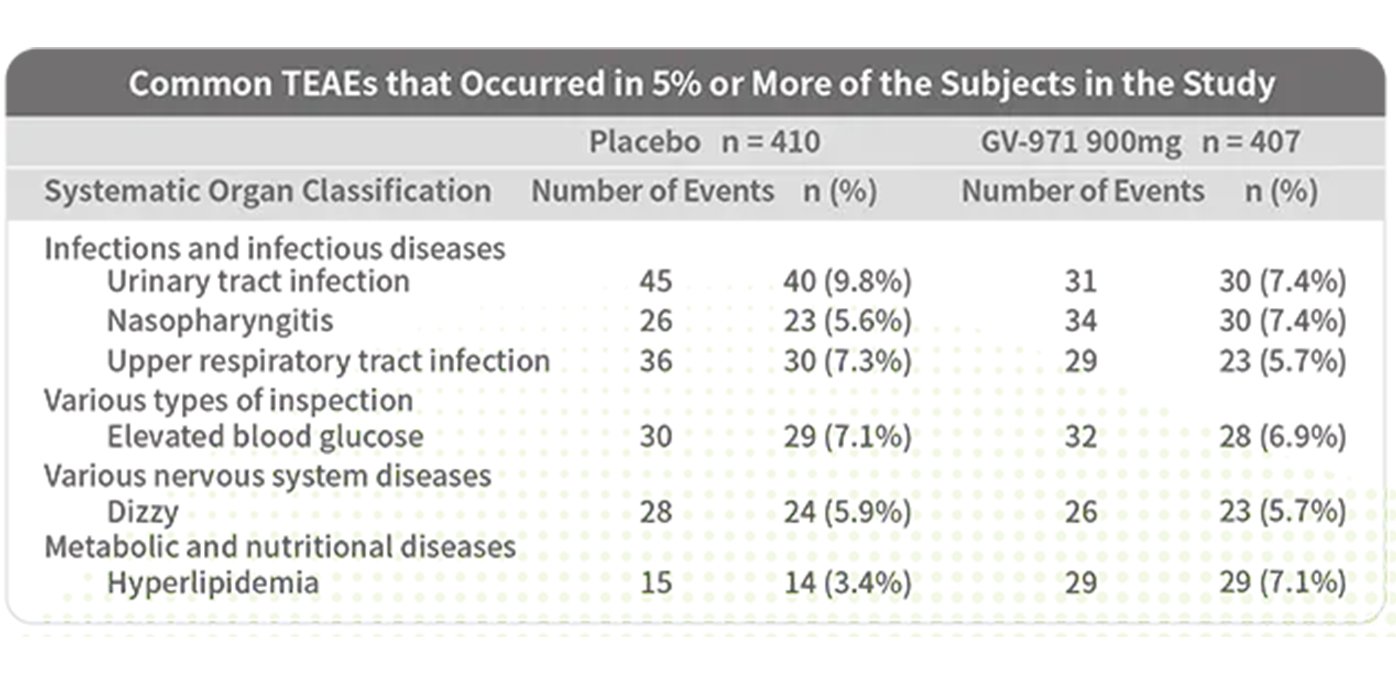
Good tolerability: the incidence of adverse events was comparable between the GV-971 group (75.4%) and the placebo group (73.9%)
1. Data on file
2. Alzheimers Res Ther. 2021 Mar 17;13(1):62.
3. ClinicalTrials.gov, NCT02293915
A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia

Xiao S, et al. Alzheimers Res Ther. 2021;13(1):62.
-

GV-971 Officially Launched in China on 29 December 2019
-


Prof. Shifu Xiao
Mental Health Center Affiliated to Shanghai Jiao Tong University School of Medicine
Leading primary investigator of GV-971 Phase III study in China


"There are only few drugs available to treat Alzheimer's disease, and none can delay or prevent progression of the disease. The results of the Phase 3 clinical study showed rapid onset of efficacy of Sodium Oligomannate within 4 weeks, and that patients' cognitive function continued to improve. The treatment was safe during the 36-week clinical trial."
-


Prof. Zhenxin Zhang
Professor of Neurology at Peking Union Medical College Hospital in Beijing
Leading principal investigator of GV-971 Phase III study in China


"I have been doing research on Alzheimer's disease for 50 years, participated in multiple global multi-center studies of multiple drugs, and have never found a satisfactory treatment for Alzheimer's disease. The result of the 9-month trial of Sodium Oligomannate is exciting. We finally see hope and dawn. I am sincerely happy for the patients and their families."
-
-

Revealed at the 11th CTAD Conference in Barcelona, 25th October 2018
Results of GV-971 China Phase III study
-


Jeffrey Cummings, MD
World-renowned neurologist
Winner of the Bengt Winblad Lifetime Achievement Award 2018 from National Alzheimer’s Association of America
Professor of Brain Science at the University of Nevada Las Vegas


"The trial of GV-971 consistently showed a cognitive benefit, it has promise as a new therapy for Alzheimer’s disease."
-


Rachel Schindler, MD
Expert in Alzheimer’s disease
President of Schindler Neurosciences Consulting Group
Previously Vice President of Pzfier Neurosciences


"These are the most inspiring and uplifting Phase III clinical results in more than a decade."
-


Eric Reiman, MD
Eecipient of the Potamkin Prize
Executive Director of Banner Alzheimer’s Institute


"I am encouraged by the cognitive improvement, safety and tolerability associated with Sodium Oligomannate in this initial clinical trial, as well as the potential to diversify the portfolio of promising treatments for our affected patients and families."
-





