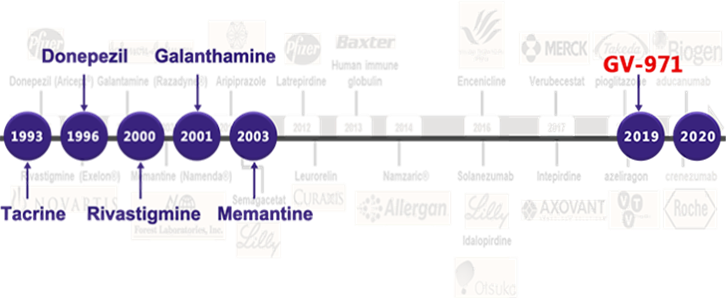
GV-971 (Sodium Oligomannate) is a first-in-class new drug for the treatment of Alzheimer's disease (AD) successfully developed by the research team led by Professor Meiyu-Geng, Deputy Director of the Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences(CAS), following persistent hard work over 22 years with the efforts successively made by Ocean University of China, the SIMM, and Shanghai Green Valley Pharmaceutical Co. Ltd. It is the world's first new drug for the treatment of Alzheimer's disease targeting the brain-gut axis. On November 2, 2019, GV-971 was approved by the NMPA through the priority review and approval process "for mild to moderate Alzheimer's disease and improving cognitive function in patients".
-
1997
-
2006
-
2008
-
2009
-
2013
-
2014
-
2018
-
2019
-
2020
-
2021
-
2022
-
The study team discovered the active substance of GV-971 from marine brown algae
-
IND approval obtained from the NMPA
-
Phase I clinical study completed
-
Global development license obtained by Green Valley
-
Phase II clinical study completed
-
Phase III clinical study initiated
-
On June 22, the last patient out (LPO)
On July 17, Phase III clinical study unblinded
On October 18, the NDA submitted
On November 14, the NDA accepted by CDE
-
In Sep. mechanism of action published
On November 2, the NDA approved
On December 29, marketing of the new drug in China
-
On April 2, the IND of global phase III clinical trial approved in the U.S. by FDA
-
On February 4, the first patient dosed globally for GP3
On August 2, the first patient dosed in Greater China for GP3
On December 16, FDA approved IND application for GV-971 global multi-center phase-II clinical trial in the treatment of Parkinson's disease
-
On January 13, 2022, the US Food and Drug Administration issued a formal decision letter to approve the Investigational New Drug (IND) application for the international multi-center phase-II clinical study of GV-971 in Parkinson’s disease– the IND effective date is December 16, 2021.
Received funding from “863 Program”, National Natural Science Foundation of China, “973 Program”, National Science and Technology Major Project of China for “Significant New Drugs Development”, Strategic Priority Research Program of Chinese Academy of Sciences (Class A) and the Shanghai Science and Technology Program, etc.
-


GV-971 Gets Included in China National Reimbursement Drug List
-

GV-971 Sodium Oligomannate Capsules NMPA Class I new drug
-


Green Valley Obtains IND Approval from U.S. FDA for Sodium Oligomannate’s Global Phase II Clinical Trial in Treating Parkinson’s Disease
-


GV-971 pre-clinical study revealed a novel mechanism of action targeting brain-gut axis with results featured on the cover of Cell Research
-


GV-971 was approved for marketing

The "Green Memory" global multi-center phase III clinical study of GV-971 is an in-depth research on the etiological mechanism of action to explore the potential for long-term disease modification
Relevant Literature Interpretation
-

 2022/05/13
2022/05/13Announcement on Early Termination of GV-971 Global Multi-Center Phase III Clinical Trial
GV-971
-

 2022/01/13
2022/01/13Green Valley Obtains IND Approval from U.S. FDA for Sodium Oligomannate’s Global Phase II Clinical Trial in Treating Parkinson’s Disease
GV-971
-

 2021/12/03
2021/12/03GV-971 Gets Included in China National Reimbursement Drug List
GV-971
-

 2021/06/16
2021/06/16GV-971 Global Phase III Clinical Trial Officially Initiates in Greater China to Clarify Its Potential in Changing Disease Course
GV-971
-

 2021/06/04
2021/06/04Nature Index-China: Alzheimer’s drug trial explores gut-brain link
GV-971