"Green Memory" Phase-III Global Multi-Center Clinical Study
(ClinicalTrails.gov Identifier:NCT04520412)
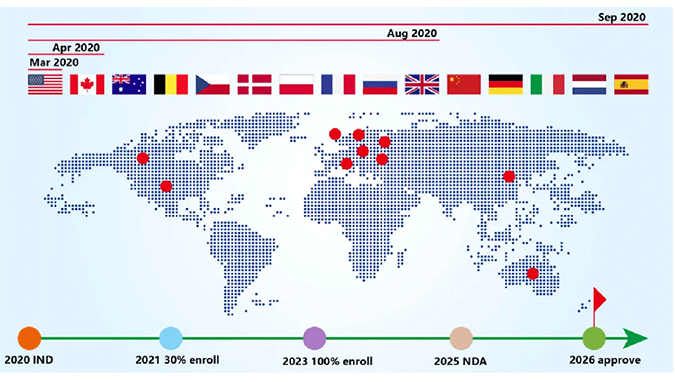
The Largest Single Clinical Trial in AD in the World
Successively approved by regulatory authorities in 9 countries/regions including the US (FDA), Canada, France, as well as Chinese mainland and Taiwan, etc.
Approximately 200 clinical sites in 14 countries and regions, including North America, Europe, and China
Involving 2046 patients with mild to moderate AD
Efficacy and safety observation for a total of 18 months, including 52-week double-blind + 26-week open-label
Inclusion of disease progression related biomarkers and microbiota testing to determine the disease –modifying potential of the drug
52-week double-blind study scheduled to be completed in 2025, followed by NDA in Europe and the US
GV-971 Global Phase III Clinical Trial Progress
-
Year 2020
-
April
IND approval of global phase III clinical trial from the U.S. FDA obtained
-
October
Global patient enrollment initiated
-
-
Year 2021
-
February
The first patient dosed worldwide and NA investigator meeting held
-
May
The first site initiated in China and Greater China investigator meeting held
-
August
The first patient dosed in Greater China
-
52-week double-blind period + 26-week open-label period
Efficacy and safety observation for a total of 18 months
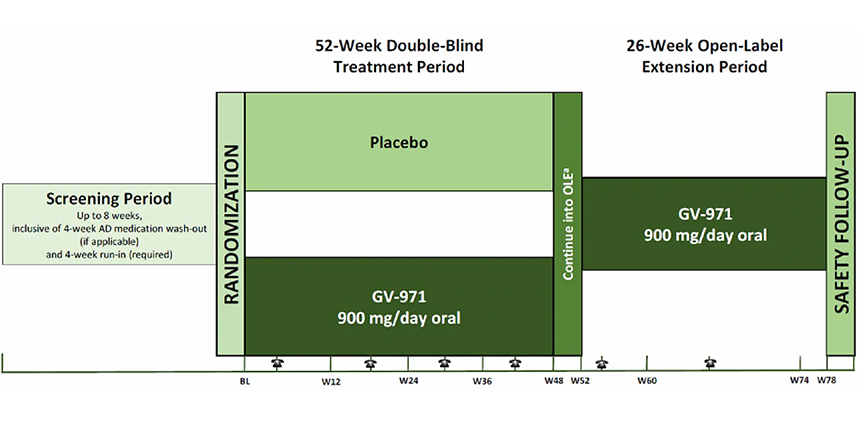
w=week
a.The patient who completes the double-blind period can continue to be enrolled in the open-label period.
Recruiting
Standards
Age 50-85
Mild to moderate Alzheimer’s disease (MMSE score 11-24)
Haven’t been enrolled in the standard treatment of Alzheimer’s disease
More information about the clinical sites
Please visit the page on clinicaltrials.gov:

Patients recruitment website of Green Memory study:
Collaborating with 13 top clinical trial service providers in the world
Ensuring the quality of the clinical trial