A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia
Abstract:
Background: New therapies are urgently needed for Alzheimer’s disease (AD). Sodium oligomannate (GV-971) is a marine-derived oligosaccharide with a novel proposed mechanism of action. The first phase 3 clinical trial of GV-971 has been completed in China.
Methods: We conducted a phase 3, double-blind, placebo-controlled trial in participants with mild-to-moderate AD to assess GV-971 efficacy and safety. Participants were randomized to placebo or GV-971 (900 mg) for 36 weeks. The primary outcome was the drug-placebo difference in change from baseline on the 12-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog12). Secondary endpoints were drug-placebo differences on the Clinician’s Interview-Based Impression of Change with caregiver input (CIBIC+), Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) scale, and Neuropsychiatric Inventory (NPI). Safety and tolerability were monitored.
Results: A total of 818 participants were randomized: 408 to GV-971 and 410 to placebo. A significant drug-placebo difference on the ADAS-Cog12 favoring GV-971 was present at each measurement time point, measurable at the week 4 visit and continuing throughout the trial. The difference between the groups in change from baseline was − 2.15 points (95% confidence interval, − 3.07 to − 1.23; p < 0.0001; effect size 0.531) after 36 weeks of treatment. Treatment-emergent adverse event incidence was comparable between active treatment and placebo (73.9%, 75.4%). Two deaths determined to be unrelated to drug effects occurred in the GV-971 group.
Conclusions: GV-971 demonstrated significant efficacy in improving cognition with sustained improvement across all observation periods of a 36-week trial. GV-971 was safe and well-tolerated.
Trial registration: ClinicalTrials.gov, NCT02293915. Registered on November 19, 2014
Keywords:sodium oligomannate, efficacy, safety, Alzheimer’s disease, clinical trial
Link to Full Text:
https://alzres.biomedcentral.com/articles/10.1186/s13195-021-00795-7
Xiao S, Chan P, Wang T, et al. Alzheimers Res Ther.2021, Mar 17;13(1):62.
Research Explanation
A research paper was published on the international peer reviewed medical journal Alzheimer’s Research & Therapy by Professor Xiao Shifu from the Shanghai Mental Health Center, Professor Zhang Zhenxin from Peking Union Medical College Hospital, and Professor Geng Meiyu from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences and other corresponding authors. It’s about a phase III clinical trial to evaluate the efficacy and safety of GV-971 on mild to moderate Alzheimer’s patients. Previously the phase II clinical trial demonstrated that GV-971 was safe and well tolerated. The phase III clinical trial demonstrated that GV-971 (900 mg) statistically improved cognitive function in mild-to-moderate AD patients. Aged 50-85 years subjects with mild-to-moderate AD were assigned randomly (1:1 ratio), to receive GV-971 (450 mg, b.i.d.) or placebo for 36 weeks. The primary efficacy endpoint was the drug–placebo difference in the change from baseline on the ADAS-Cog12l. A significant drug-placebo difference on the ADAS-Cog12 favoring GV-971 was present at each measurement time point, measurable at the week 4 visit and continuing throughout the 36-week trial, while treatment-emergent adverse event incidence was comparable between active treatment and placebo.
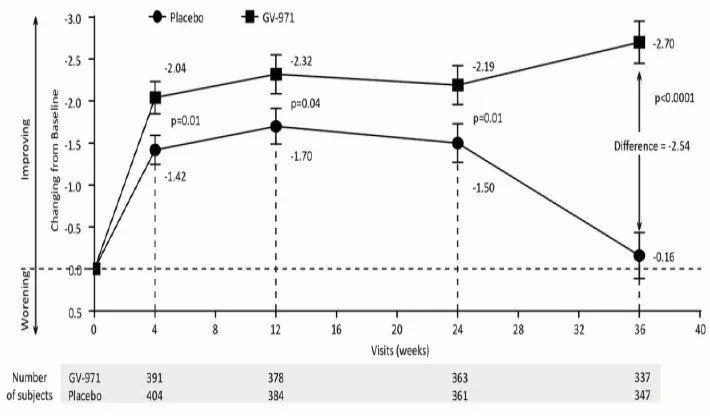
Figure (citied from Alzheimer's Research & Therapy)
Mean ADAS-Cog12 score change from baseline at weeks 4, 12, 24, and 36 (observed value).
The mean change from baseline to week 36 on the ADAS-Cog12 (with scores ranging from 0 to 75 and higher scores indicating greater impairment) by full analysis set was showed. Error bars represent standard errors (SE). p values are obtained from the Wilcoxon rank sum tests.
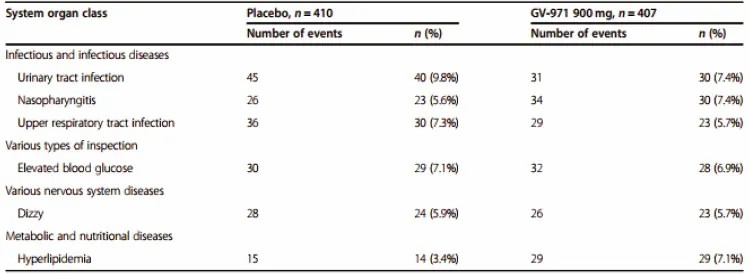
Table: TEAEs that occurred in 5% or more of the subjects in the study (citied from Alzheimer's Research & Therapy)
Note: By definition, TEAE, treatment-emergent adverse event, refers to any adverse event that has occurred, regardless of its relationship with the drug study, during the period from the drug administration of the first double-blind study to 28 days after the drug administration of the last double-blind study, or to the early termination date of subject withdrawal before V6.
Non-clinical studies have confirmed that GV-971 can reduce neuroinflammation by regulating the brain-gut axis and reducing peripheral inflammation, and can also directly bind with amyloid β-protein (Aβ), thereby reducing Aβdeposition in the brain. The phase III clinical trial confirmed the efficacy and safety of the drug, evaluated the relationship between benefits and risks, and eventually provided a sufficient basis for the review of the drug's marketing application. From week 4 onwards, there was significant and consistent improvement on cognitive function of the GV-971 group throughout the trial until week 36. At the same time, there was no significant difference in the incidence of adverse events compared with the placebo group.Since GV-971’s phase II and phase III clinical studies were all conducted in China, its efficacy and safety in other populations remains unclear. That’s why the sponsor is currently executing a global multi-center phase III clinical trial, the design of which extends the double-blind trial period from 9 months to 12 months, followed by a 6-month open period – a total of 18 months for efficacy and safety observations. At the same time, the trial includes exploratory indicators of biomarkers (including gut microbiota, gut metabolites, AD biomarkers, inflammatory cells, etc.), so the mechanism of action of GV-971 on the brain-gut axis is expected to be further verified. To date, the application for GV-971’s international multi-center clinical trial has been approved by the US Food and Drug Administration (FDA) as well as drug administrations in nine countries and regions such as Canada, France, and the Chinese Mainland, and the first drug administration of the international trial was successfully completed on February 3, 2021.
Previously, there were only two types of drugs that could be used to improve the cognitive function of AD patients in clinical practice, and both of them were neurotransmitter agents, which are unable to inhibit disease progression. So there were very limited options for AD patients. However, the phase III clinical trial confirmed that Sodium Oligomannate (GV-971), as a drug with a new mechanism of action, can significantly improve the cognitive function of patients with mild to moderate AD and potentially alter the course of the disease , while being safe and well-tolerated.In recent years, scientists have also begun to pay attention to the important role that gut microbiota plays in the occurrence and development of AD. New evidence has emerged in the field to support the hypothesis that the interaction between gut microbiota dysbiosis, neuroinflammation, Aβ deposition and tau phosphorylation will accelerate the progression of AD. Non-clinical studies have shown that the therapeutic effects of GV-971 on gut microbiota dysbiosis, neuroinflammation and Aβ deposition can improve the pathological cascade response, therefore addressing symptoms, potentially delaying disease progression, and bringing long-term benefits to patients. GV-971 was approved for market launch in China at the end of 2019, providing new option for the treatment of AD, while presenting new inspirations for basic research and new drug development in the field.