AD/PD 2022: Deeply Explores the Gut Microbiota’s Implications for PD Pathogenesis and Treatment
On March 20, 2022, the AD/PD 2022 International Conference on Alzheimer's and Parkinson's Diseases formally concluded in Barcelona, Spain. Held in a hybrid form spanning both online and offline and attended by neurologists and researchers across the globe, the six-day academic conference focused on topics including newest research breakthroughs, drug research and development, early diagnosis, and clinical studies around Alzheimer’s and Parkinson’s diseases and related neurological disorders.
As the second most common neurodegenerative disease after Alzheimer’s disease (AD), the pathogenesis of Parkinson’s disease (PD) is still inconclusive, but it is generally believed that the disease is associated with α-synuclein aggregation, neuroinflammation and oxidative stress, and mitochondrial dysfunction. In recent years, more and more studies have shown that the gut microbiota is highly correlated with PD’s occurrence and development.
“Emerging data highlight the role of the gut microbiome in PD, which were proposed to regulate the brain function through multiple ways.” Stated Prof. Philip Scheltens, Director of the Alzheimer Center at Amsterdam UMC, during AD/PD 2022.
Philip Scheltens delivers opening remarks at Green Valley special symposium
That came as the opening remarks for Gut Microbiota and Parkinson’s Disease: Implications for Pathogenesis and Treatment, a symposium hosted by Green Valley, where three Chinese and international experts shared insights into PD’s mechanism of action, pharmacological therapy, and non-pharmacological therapy.
In a presentation titled Personalized Medicine in Parkinson’s Disease and The Role of The Gut-Brain Axis, Prof. Teus Van Laar, Medical Director of the Parkinson Expertise Center at the University of Groningen in the Netherlands, talked about studies on the association between the gut-brain axis and PD.
For example, the research team headed by Timothy R. Sampson from the California Institute of Technology published a study on Cell in 2016, which was conducted on a PD mouse model. The mice had an alpha-synuclein overexpression that’s specific to PD in their brain, and showed motor symptoms. When the research team cleaned the gut of the mice completely with antibiotics and laxatives, their parkinsonian symptoms were alleviated. However, when they transplanted fecal microbiota from PD patients into the same mice, the animals once again displayed motor dysfunction, as compared to no parkinsonian symptoms in animals colonized with microbes from healthy controls. Together, these results suggest that gut microbes may play a critical and functional role in the pathogenesis of synucleinopathies such as PD1.

Prof. Teus Van Laar presents Personalized Medicine in Parkinson’s Disease and The Role of The Gut-Brain Axis.
Speaking after Prof. Van Laar, Prof. Geng Meiyu, Academic Director General of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, presented her report titled Mechanistic Insights Underlying GV-971 Counteracting Parkinson's Disease in Animal Models. The presentation explored whether GV-971 can counteract PD, which has a similar pathogenesis like AD, by targeting the gut-brain axis.
Being the world’s first AD drug that targets the gut-brain axis, GV-971 reduces peripheral and central inflammation by reconditioning the gut microbiota and inhibiting the abnormal increase of gut microbiota-derived metabolites. Results of GV-971’s China phase-3 clinical study demonstrated that the novel drug had fast-acting and consistent improvement in the cognitive function of patients, while being safe and well tolerated with the incidence of adverse events comparable to the placebo group2. And in 2021, a review article published in international journal The Lancet pointed out that globally, only two marketed drugs are capable of altering the course of AD, and GV-971 is one of them3. On such a basis, the research team at Green Valley Research Institute focused on the common pathological mechanism of neurodegenerative diseases to conduct preclinical studies on the treatment of PD with GV-971.
“All these non-clinical model studies reveal the unique mechanism of action of GV-971. By targeting the gut microbiota, the drug can reconstitute gut microbiota dysbiosis in several PD animal models, and reduce α-synuclein deposition in both intestine and brain as well as reduce neuroinflammation.” Noted Prof. Geng Meiyu in her presentation. “Consequently, GV-971 can protect dopaminergic neurons, and improve both motor and non-motor symptoms. And we also found that a better effect can be observed with longer treatment.”
Based on these study results, the United States Food and Drug Administration (FDA) approved GV-971’s proposed global phase-2 clinical study in treating PD on December 16, 2021. The study will be a 36-week, multi-center, randomized, double-blind, parallel-controlled trial, plus a 36-week open-label extension trial. It plans to enroll 300 patients with early-stage PD, and will be conducted at 30 clinical centers in North America and Asia Pacific to evaluate the efficacy and safety of GV-971 in the treatment of early-stage PD.
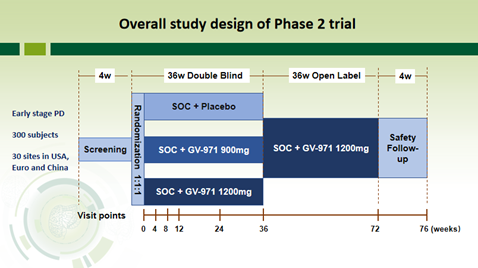
USFDA has approved GV-971’s proposed global phase-2 clinical study in treating PD.
Prof. Geng said that the primary endpoint of the global clinical study is motor function, while changes in non-motor symptom-related scales will be evaluated as well. In addition, investigators will also evaluate exploratory endpoints, including biomarkers related to gut microbiota dysbiosis.
Besides GV-971’s effects in reducing neuroinflammation by targeting the gut-brain axis, more and more studies have also proven that neuroinflammation is closely associated with neurodegenerative diseases including AD and PD. In the last presentation of the Green Valley special symposium, Chen Shengdi, Professor of Neurology at Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, shared his report titled Non-Pharmacological Therapy for Neurodegenerative Diseases, introducing cognitive and Tai Chi training’s positive effects on patients with cognitive impairment and potential for reducing inflammation.
The research team headed by Prof. Chen performed two clinic projects, using Tai Chi training for early-stage PD patients. The first study observed one-year training effects for improvements of motor and non-motor symptoms, including Tai Chi and Brisk Walking training. The second study observed whether long-term Tai Chi training could delay the progression of PD over an average course of three years. The research team found that the during the period, more patients needed adding usage of 18 PD drugs and had higher UPDI-3 rating scales in the control group than those in the Tai Chi group, therefore indicating that Tai Chi training could delay the progression of early-stage PD. In addition, the team also found that inflammatory cytokines decreased significantly in patients after Tai Chi training compared to the control group.
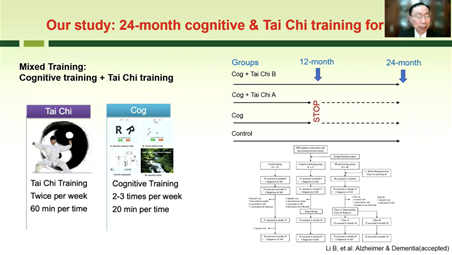
Prof. Chen Shengdi shares report Non-Pharmacological Therapy for Neurodegenerative Diseases.
Prof. Chen summarized: “Our study results show that cognitive training can improve the cognition in MCI (mild cognitive impairment) patients, Tai Chi can enhance cognitive training effects on delaying cognitive decline in MCI patients, and Tai Chi training can improve the motor symptoms and cognitive function of PD patients and might delay the progression of PD. The mechanism of cognitive and Tai Chi training effects may be related to increased neuroplasticity, decreased inflammation and others.”
References:
1. Timothy R. Sampson et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell, December 2016 DOI: 10.1016/j.cell.2016.11.018
2. Xiao, S., Chan, P., Wang, T., Hong, Z., Wang, S., Kuang, W., ... & Zhang, Z. (2021). A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimer's research & therapy, 13(1), 1-11.
3. Philip Scheltens, Bart De Strooper, Miia Kivipelto, et al. Alzheimer’s disease. The Lancet. 2021, April 24, 397(10284):1577-1590.






